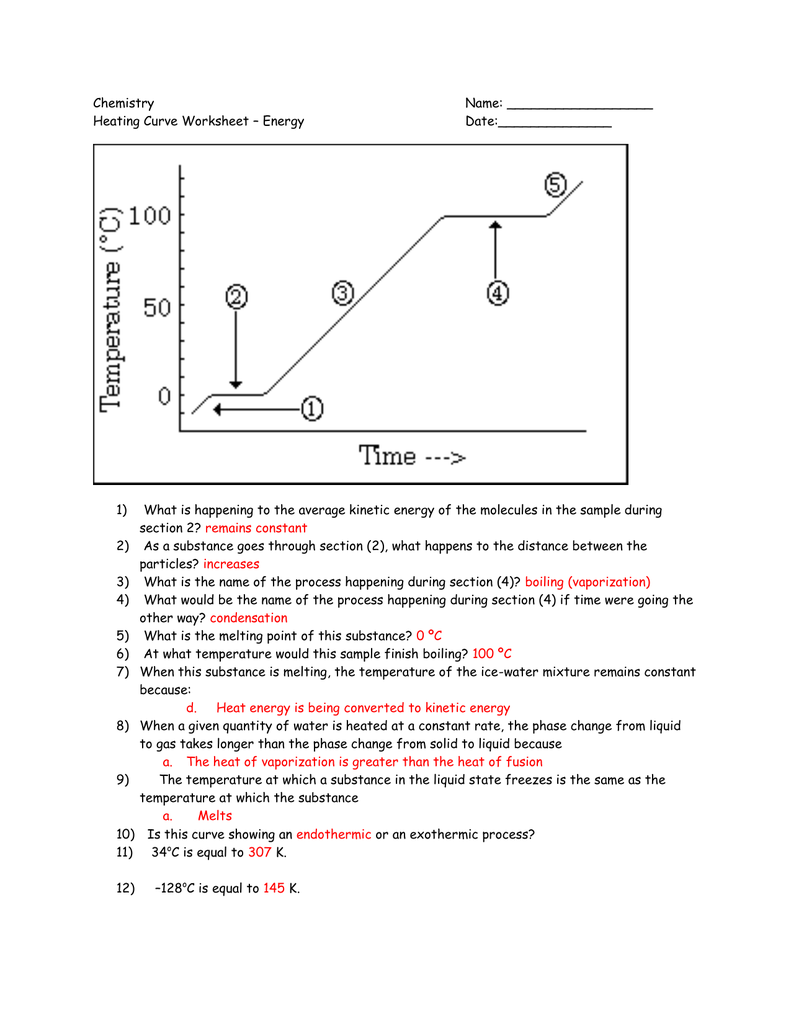
Heating And Cooling Curve Of Paradichlorobenzene Lab Answers . freezing point is best determined by the use of cooling curves. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. Heat steam from 100 °c to 120 °c. Q = m × c × δ t (see previous chapter on thermochemistry). the graph below represents the relationship between temperature and time as heat is added to a sample 6. change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. The heat needed to induce a given change in phase is given by q = n × δ h. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. heat water from 0 °c to 100 °c. The heat needed to change the temperature of a given substance (with no change in phase) is:
from learningschoolvaljevuzd.z22.web.core.windows.net
in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. Q = m × c × δ t (see previous chapter on thermochemistry). change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. heat water from 0 °c to 100 °c. Heat steam from 100 °c to 120 °c. freezing point is best determined by the use of cooling curves. The heat needed to change the temperature of a given substance (with no change in phase) is: the graph below represents the relationship between temperature and time as heat is added to a sample 6. The heat needed to induce a given change in phase is given by q = n × δ h.
Worksheet On Heating And Cooling Curves
Heating And Cooling Curve Of Paradichlorobenzene Lab Answers in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. the graph below represents the relationship between temperature and time as heat is added to a sample 6. heat water from 0 °c to 100 °c. Q = m × c × δ t (see previous chapter on thermochemistry). The heat needed to change the temperature of a given substance (with no change in phase) is: change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. freezing point is best determined by the use of cooling curves. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. The heat needed to induce a given change in phase is given by q = n × δ h. Heat steam from 100 °c to 120 °c.
From studyschoolburman.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curve Of Paradichlorobenzene Lab Answers in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. The heat needed to change the temperature of a given substance (with no change in phase) is: Heat steam from 100 °c to 120 °c. change the solid paradichlorobenzene into the liquid state by slowly heating. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From amiahgokeestes.blogspot.com
Use the Heating Curve Below to Answer the Following Questions Heating And Cooling Curve Of Paradichlorobenzene Lab Answers The heat needed to change the temperature of a given substance (with no change in phase) is: heat water from 0 °c to 100 °c. Q = m × c × δ t (see previous chapter on thermochemistry). Heat steam from 100 °c to 120 °c. in this laboratory investigation, you will measure the change in temperature as. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From lessonlibraryfettes.z22.web.core.windows.net
Heating Curve Calculations Worksheet Heating And Cooling Curve Of Paradichlorobenzene Lab Answers Heat steam from 100 °c to 120 °c. change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. The heat needed to induce a given change in phase is given by q = n × δ h. in this laboratory investigation, you will measure the. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From printablefullfared.z19.web.core.windows.net
Heating Curve Lab Answers Heating And Cooling Curve Of Paradichlorobenzene Lab Answers heat water from 0 °c to 100 °c. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. The heat needed to induce a given change in phase is given by q = n × δ h. in this laboratory investigation, you will measure the. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From printablefullfared.z19.web.core.windows.net
Heating Curve Lab Answers Heating And Cooling Curve Of Paradichlorobenzene Lab Answers change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. The heat needed to change the temperature of a given substance (with no change in phase) is: Heat steam from 100 °c to 120 °c. Q = m × c × δ t (see previous chapter. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From www.scribd.com
Heating and cooling curve PDF Heating And Cooling Curve Of Paradichlorobenzene Lab Answers Q = m × c × δ t (see previous chapter on thermochemistry). The heat needed to induce a given change in phase is given by q = n × δ h. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. Heat steam from 100 °c. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From studylib.net
Heating Curve Lab Heating And Cooling Curve Of Paradichlorobenzene Lab Answers The heat needed to induce a given change in phase is given by q = n × δ h. heat water from 0 °c to 100 °c. freezing point is best determined by the use of cooling curves. change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From excelkayra.us
Heating Cooling Curve Worksheet Answer Key Kayra Excel Heating And Cooling Curve Of Paradichlorobenzene Lab Answers Q = m × c × δ t (see previous chapter on thermochemistry). in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. freezing point is best determined by the use of cooling curves. The heat needed to change the temperature of a given substance (with. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From learningfullmaurer.z1.web.core.windows.net
Heating Curve Lab Answers Heating And Cooling Curve Of Paradichlorobenzene Lab Answers in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. heat water from 0 °c to 100 °c. The heat needed to induce a given change in phase is given by q = n × δ h. the graph below represents the relationship between temperature. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From printablefullfared.z19.web.core.windows.net
Heating Curve Lab Answers Heating And Cooling Curve Of Paradichlorobenzene Lab Answers in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. Heat steam from 100 °c to 120 °c. The heat needed to induce a given change in phase is given by q = n × δ h. Q = m × c × δ t (see previous. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From materialdbhutchins.z21.web.core.windows.net
Heating Curve Lab Answers Heating And Cooling Curve Of Paradichlorobenzene Lab Answers freezing point is best determined by the use of cooling curves. heat water from 0 °c to 100 °c. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. Q = m × c × δ t (see previous chapter on thermochemistry). in this. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From www.youtube.com
How to Read and Interpret a Heating Curve or Cooling Curve YouTube Heating And Cooling Curve Of Paradichlorobenzene Lab Answers The heat needed to induce a given change in phase is given by q = n × δ h. freezing point is best determined by the use of cooling curves. heat water from 0 °c to 100 °c. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating And Cooling Curve Of Paradichlorobenzene Lab Answers Q = m × c × δ t (see previous chapter on thermochemistry). heat water from 0 °c to 100 °c. The heat needed to induce a given change in phase is given by q = n × δ h. Heat steam from 100 °c to 120 °c. the graph below represents the relationship between temperature and time. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf Heating And Cooling Curve Of Paradichlorobenzene Lab Answers heat water from 0 °c to 100 °c. Heat steam from 100 °c to 120 °c. change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From obropolox.blogspot.com
43 heating cooling curve worksheet answers Worksheet Resource Heating And Cooling Curve Of Paradichlorobenzene Lab Answers Q = m × c × δ t (see previous chapter on thermochemistry). in this laboratory investigation, you will measure the change in temperature as a hot liquid loses energy and changes to a solid. the graph below represents the relationship between temperature and time as heat is added to a sample 6. change the solid paradichlorobenzene. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From studylib.net
Heating Curve Lab Heating And Cooling Curve Of Paradichlorobenzene Lab Answers the graph below represents the relationship between temperature and time as heat is added to a sample 6. Heat steam from 100 °c to 120 °c. Q = m × c × δ t (see previous chapter on thermochemistry). heat water from 0 °c to 100 °c. in this laboratory investigation, you will measure the change in. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From db-excel.com
Heating Cooling Curve Worksheet Answers — Heating And Cooling Curve Of Paradichlorobenzene Lab Answers change the solid paradichlorobenzene into the liquid state by slowly heating the test tube over a burner flame while holding the test tube. freezing point is best determined by the use of cooling curves. Q = m × c × δ t (see previous chapter on thermochemistry). Heat steam from 100 °c to 120 °c. heat water. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating And Cooling Curve Of Paradichlorobenzene Lab Answers Heat steam from 100 °c to 120 °c. the graph below represents the relationship between temperature and time as heat is added to a sample 6. freezing point is best determined by the use of cooling curves. heat water from 0 °c to 100 °c. change the solid paradichlorobenzene into the liquid state by slowly heating. Heating And Cooling Curve Of Paradichlorobenzene Lab Answers.